
Our QMS brings all the standard capabilities every QMS has, but it is 100% adaptive. In other words, you can choose to follow the industry’s best practices Orcanos provides or take it your own way and save time on onboarding by defining your own workflows and processes.

All company stakeholders, whether that is Research and Development, Quality Assurance, Clinical, or executive management, should have minimal barriers to effective Document Control.Orcanos combines all elements into a one-stop shop for documents via an integrated Design Control process and a Quality Management System, so you can easily reduce the bottlenecks on document routing and sign-off. This single repository gives end users digital access to approved or current documents, batch release documents via the Change Control, and automates the read & understand using the Orcanos Training Module.
All company stakeholders, whether that is Research and Development, Quality Assurance, Clinical, or executive management, should have minimal barriers to effective Document Control.
Orcanos combines all elements into a one-stop shop for documents via an integrated Design Control process and a Quality Management System, so you can easily reduce the bottlenecks on document routing and sign-off. This single repository gives end users digital access to approved or current documents, batch release documents via the Change Control, and automates the read & understand using the Orcanos Training Module.
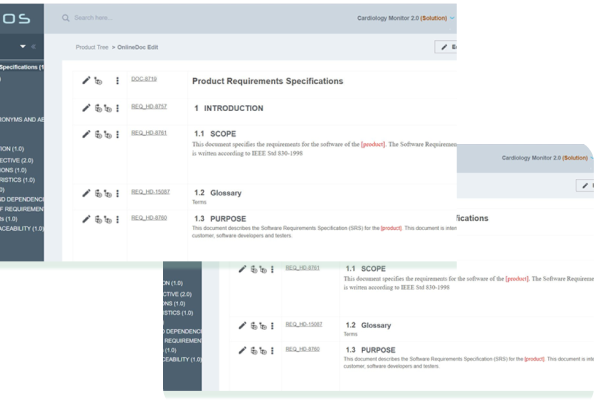

you get better compliance management with the up-to-date regulatory-wise tools in a holistic environment.

when your compliance officer is happy, you are happy too. More time to invent stuff instead of regulatory work.